In the battle against cancer, the frontlines have traditionally been drawn at the tumor itself—cutting, burning, and poisoning cells into submission. But medicine has changed. With the rise of immunotherapy, the battlefield has shifted inside the body, where the immune system is both sword and shield. And now, thanks to groundbreaking research from Japan’s National Cancer Center Research Institute, we’re beginning to understand that some of the most powerful allies in this fight may be microscopic—and living inside the human gut.
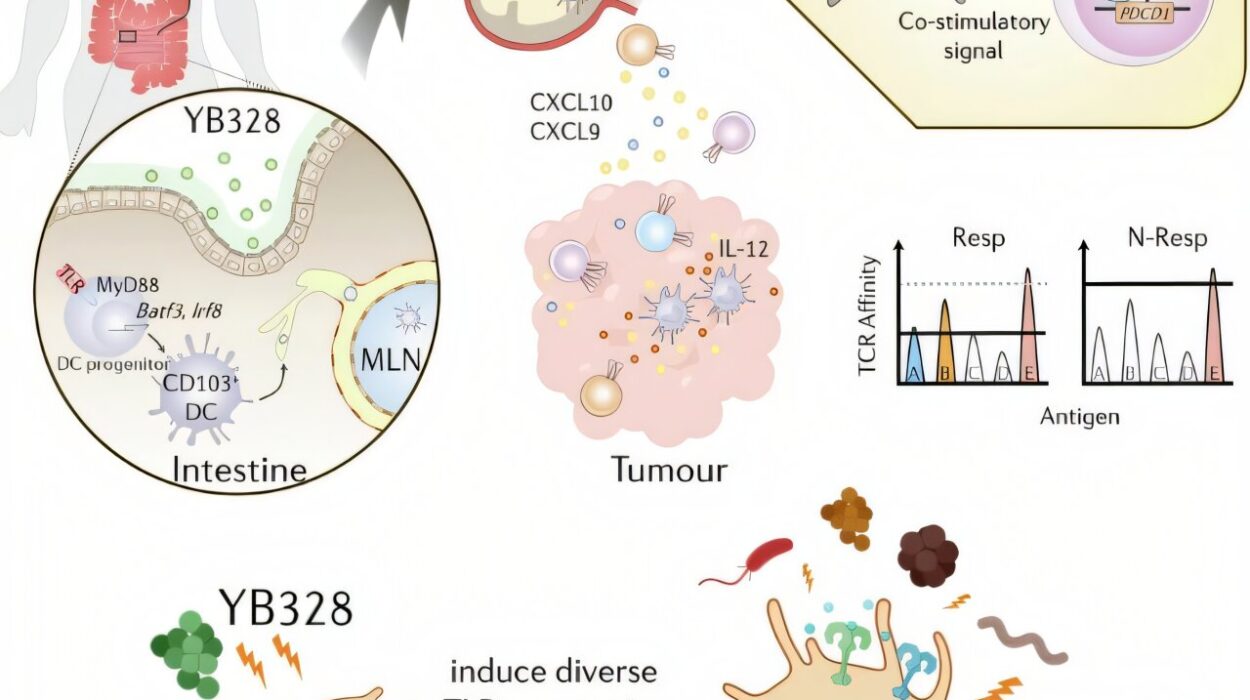
A newly discovered bacterial strain, quietly thriving in the intestines of certain cancer patients, appears to strengthen the body’s immune response in a way that dramatically improves the success of PD-1 checkpoint blockade therapies. This tiny organism, now identified as Hominenteromicrobium strain YB328, is offering a new kind of hope—not just for treatment, but for tailoring immunotherapy in ways never imagined before.
Checkpoint Blockade: Teaching the Immune System to See Again
To understand why this finding is so important, we need to step briefly into the world of checkpoint inhibitors—the cornerstone of modern immunotherapy. These drugs, such as anti-PD-1 and anti-PD-L1 monoclonal antibodies, work not by killing cancer cells directly but by releasing the brakes on the immune system. In many cancer patients, T cells are perfectly capable of destroying tumor cells, but they’re muted—restrained by molecular “checkpoints” designed to prevent autoimmune damage.
Checkpoint inhibitors disable those brakes, allowing CD8+ T cells, the body’s cytotoxic warriors, to recognize and attack cancer. But not all patients respond equally. Some achieve long-term remission; others show no improvement. The reasons behind this variability have puzzled oncologists for years.
Now, new evidence suggests that at least part of the answer may lie far from the tumor site—in the gut.
A Microscopic Messenger
Researchers at the Thompson Institute in Tokyo sought to explore whether specific gut microbes could influence how well the immune system responds to PD-1 blockade therapies. To do this, they analyzed stool samples from 50 cancer patients—15 with non-small cell lung cancer, and 35 with gastric cancer—all of whom were receiving immune checkpoint inhibitors.
Using 16S rRNA gene sequencing, they examined the microbial composition of each patient’s gut. It soon became clear that some bacteria were more than just passive residents—they were active players in immune modulation.
The standout was YB328, a strain never before isolated, but found in high abundance in the guts of patients who responded favorably to PD-1 blockade. These individuals didn’t just feel better—they lived longer without their cancer progressing. Their tumors were filled with activated T cells, and their immune systems appeared tuned for a stronger antitumor response.
From Gut to Tumor: Mobilizing the Immune Army
To understand how a gut bacterium could influence immune responses in distant organs, researchers turned to animal models. Germ-free mice—raised without any gut microbes of their own—were colonized with fecal samples from patients with and without YB328. Then, tumors were introduced, and anti-PD-1 monoclonal antibodies were administered.
The results were striking.
Mice colonized with YB328 showed slower tumor growth, increased infiltration of CD8+ T cells, and greater numbers of cytokine-producing T cells—immune cells actively fighting back against cancer. Further analysis revealed a surge in a rare but crucial type of immune cell: CD103+ CD11b− conventional dendritic cells.
These dendritic cells play an essential role in presenting antigens to T cells and priming them for attack. When YB328 was present, these dendritic cells migrated into tumors more frequently and displayed high levels of activation markers such as CD86, CD80, and MHC-I—molecules that help initiate the immune cascade.
In short, YB328 was acting like a field general, mobilizing and directing immune troops with astonishing precision.
The Shadow Side: When the Wrong Microbe Takes Over
The power of YB328 becomes even more evident when contrasted with another bacterial species, Parabacteroides vulgatus. In patients whose guts were colonized by P. vulgatus, the opposite pattern emerged: lower levels of tumor-fighting T cells, reduced PD-1+ CD8+ T cell accumulation, and shorter progression-free survival.
Even more alarmingly, in mice colonized with both YB328 and P. vulgatus, the beneficial effects of YB328 were entirely neutralized. It was as if P. vulgatus could cancel out the immune-boosting power of its beneficial counterpart. This delicate balance between “good” and “bad” microbes shows how the gut microbiome functions as a complex ecosystem—one that can either support or sabotage cancer treatment.
Not Just About Who’s There—But What They Do
Interestingly, previous studies had identified other genera—Faecalibacterium, Bifidobacterium, Enterococcus, and Akkermansia—as potentially beneficial in immunotherapy. But in this study, their presence did not significantly differ between responders and non-responders. This suggests that not all beneficial microbes act the same way in all contexts.
Rather than just cataloging which bacteria are present, scientists are now focusing on what functions these microbes perform—how they affect immune cell behavior, which molecules they secrete, and how they shape the tumor microenvironment. YB328 appears to actively reprogram the immune system, pushing dendritic cells into high gear and guiding CD8+ T cells to where they’re needed most.
Toward a New Era of Precision Microbiome Therapy
The implications of this discovery reach far beyond the lab. With reliable biomarkers still scarce, many patients undergo immunotherapy without knowing whether they’ll respond. Some suffer through side effects without benefit. YB328 offers something revolutionary—a potential predictive biomarker, and perhaps even a therapeutic agent in its own right.
Imagine a future where a simple stool test can tell doctors if a patient has YB328—and whether they’re likely to benefit from checkpoint inhibitors. Imagine using probiotics or microbial transplants to colonize patients with YB328 before immunotherapy, giving their immune systems a head start. Or designing drugs that mimic the immunostimulatory signals YB328 sends to dendritic cells.
This isn’t science fiction. It’s the next frontier of oncology and microbiology merging into a new science of precision cancer care.
The Gut–Tumor Axis: A Paradigm Shift in Cancer Medicine
What this research makes clear is that the gut microbiota is not a passive backdrop—it is an active player in cancer treatment. The microbes that inhabit our intestines don’t just digest food or make vitamins. They may help control the destiny of tumors, deciding whether immune cells are alert or asleep, whether treatment succeeds or fails.
The gut–tumor axis may soon become as central to cancer medicine as genetics and biomarkers are today. And microbes like YB328 could become key tools in a physician’s arsenal—not as drugs, but as partners.
Hope, Hidden in the Microbiome
The discovery of YB328 is more than a scientific milestone—it’s a message. That within each of us, beneath the surface, unseen and often unthought-of, there are allies waiting to be discovered. That healing may not only come from high-tech drugs or billion-dollar machines, but from something far older and more intimate—our microbiome.
As researchers around the world continue to decode the complex language between microbes and immune cells, one thing becomes clearer: the future of cancer therapy may begin in the gut. And in that future, a newly named bacterium from Tokyo could help turn the tide for patients whose options once seemed limited.
In the war against cancer, YB328 might just be the whisper that turns into a roar.
More information: Nina Yi-Tzu Lin et al, Microbiota-driven antitumour immunity mediated by dendritic cell migration, Nature (2025). DOI: 10.1038/s41586-025-09249-8